Revolutionary Huntington’s Disease Treatment: First-Ever Therapy Slows Progression by 75%
In a historic medical breakthrough, doctors at University College London (UCL) have successfully treated Huntington’s disease for the first time. This devastating genetic condition, which resembles a combination of dementia, Parkinson’s, and motor neurone disease, has long been considered untreatable. The revolutionary gene therapy has shown remarkable results, slowing disease progression by 75% – meaning what would typically be a year of decline now takes four years.
How the Groundbreaking Therapy Works
The treatment represents a significant leap forward in Huntington’s disease treatment approaches. Unlike previous therapies that only managed symptoms, this new approach targets the root cause of the disease – the faulty huntingtin gene that produces toxic proteins damaging brain cells.
Gene Therapy Mechanism
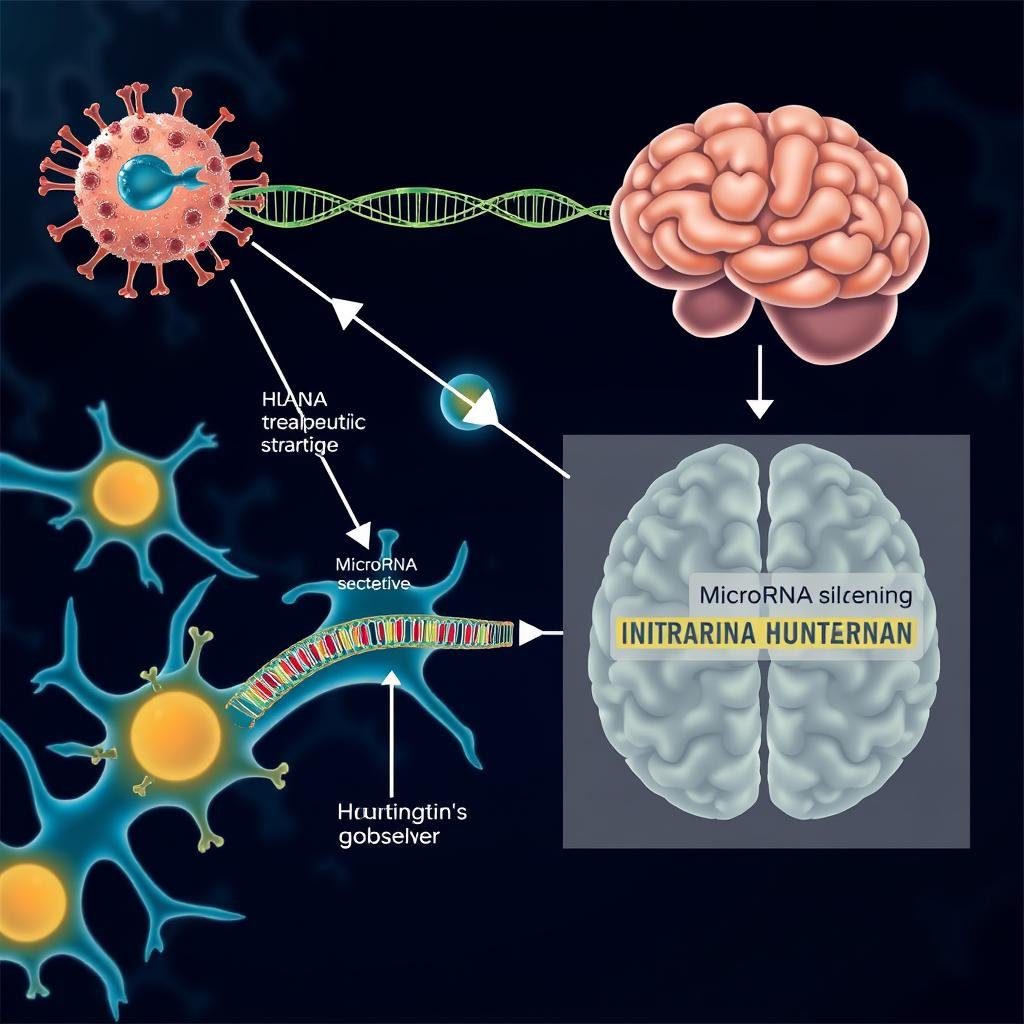
The therapy utilizes a sophisticated gene silencing technique delivered through a delicate 12-18 hour brain surgery. A harmless virus carries specially designed DNA into the brain, which prompts cells to produce microRNA that effectively silences the faulty huntingtin gene.
This one-time treatment appears to have long-lasting effects, potentially for life, as brain cells are not frequently replaced. The therapy works by essentially turning off the production of the harmful protein that causes progressive brain damage.
“We now have a treatment for one of the world’s more terrible diseases. This is absolutely huge. I’m really overjoyed.”
The surgical procedure involves infusing the virus very slowly through a micro-catheter into two separate brain regions. While complex, this approach allows for precise delivery of the therapeutic agent directly to the affected areas of the brain.
Promising Trial Results
The trial involved 29 patients treated in the UK and US over a three-year period. While the data has not yet been peer-reviewed, the preliminary results are extremely promising:
75% Slower Progression
Patients receiving the high dose of the therapy experienced a 75% reduction in disease progression compared to standard care. This dramatic slowdown means that what would typically be one year of decline now takes four years.
Preserved Mobility
Participants maintained their ability to walk and perform daily activities for significantly longer periods. One patient was even able to return to work after previously having to retire due to the disease.
Reduced Brain Cell Death
Biological markers showed clear evidence that neurons were being spared. Levels of neurofilaments – indicators of cell death – were significantly lower in the treatment group compared to those receiving standard care.
Key Stat: 75,000 Affected Patients
Approximately 75,000 people have Huntington’s disease in the UK, US, and Europe, with many more carrying the gene that will eventually develop into the condition.
Safety Profile and Treatment Accessibility
Managing Side Effects
While the treatment has shown remarkable efficacy, some side effects were observed during the trial. These primarily included inflammation that caused headaches and confusion in some patients. However, researchers reported that these effects either resolved on their own or were successfully managed with steroids.
Overall, the treatment was considered safe, with patients tolerating the complex surgical procedure well. The benefits of slowing disease progression significantly outweighed the manageable side effects for trial participants.
Availability Timeline
The therapy, developed by the company uniQure, is expected to follow a structured approval and rollout process:
Future Implications and Research Directions
The success of this gene therapy opens exciting new possibilities for Huntington’s disease treatment and research. Scientists are already planning to test early preventive treatment for people who carry the Huntington’s gene but do not yet have symptoms (stage zero Huntington’s).
This breakthrough could fundamentally transform treatment approaches, potentially preventing symptoms from occurring if the therapy is administered at an earlier stage. The dramatic impact raises the possibility that with early intervention, some patients might never experience the devastating effects of the disease.
The success of this approach may also inspire similar gene therapy strategies for other neurodegenerative conditions. The technology and delivery methods developed for this treatment could potentially be adapted to address other genetic brain disorders.
Impact on Genetic Testing
With an effective treatment now available, more people from families affected by Huntington’s may choose to undergo genetic testing. Previously, only about 20% of those at risk opted for testing since there were no treatments to slow progression.

Life-Changing Impact for Patients
“This could fundamentally transform treatment for Huntington’s and inspire further advances for more patients.”
For those living with Huntington’s disease, this breakthrough offers unprecedented hope. The ability to slow disease progression by 75% means patients could maintain independence, continue working, and enjoy quality time with loved ones for significantly longer periods.

The treatment’s impact extends beyond physical symptoms. By preserving cognitive function and delaying the onset of dementia-like symptoms, patients may maintain their sense of self and personal relationships for many additional years.
Learn More About Huntington’s Disease Treatment Options
Stay informed about the latest developments in Huntington’s disease treatment and find specialized care near you.
A New Era in Huntington’s Disease Treatment
The successful treatment of Huntington’s disease marks a historic turning point in the fight against this devastating condition. After decades of research with limited progress, this breakthrough offers genuine hope to patients and families affected by the disease.
While challenges remain regarding treatment accessibility and cost, the scientific achievement cannot be overstated. For the first time, medical science has demonstrated the ability to significantly slow the progression of a condition previously considered untreatable.

As research continues and the treatment moves toward wider availability, thousands of patients worldwide may soon benefit from this revolutionary approach to Huntington’s disease treatment.
Support Huntington’s Disease Research
Help advance research and support for those affected by Huntington’s disease.






